Neuralink Brain Chip: The Cyborg Revolution Has Begun
1. Introduction — What is the Neuralink brain chip and Why is it No Longer Science Fiction
The promise of directly linking the human mind to a machine—a concept once relegated to dystopian science fiction—is now a tangible reality, spearheaded by companies like Neuralink. The Neuralink brain chip is arguably the most well-known product in the emerging field of neurotechnology, a compact, fully implantable brain-computer interface technology (BCI) designed to read electrical signals from the brain and translate them into digital commands. This is far more than mere technological hype; it represents the birth of the “real BCI cyborg”—individuals regaining control over external devices, not through muscle movement, but through the sheer power of thought.
Neuralink, founded in 2016 by Elon Musk and a team of neuroscientists and engineers, has quickly gained global attention for its ambitious goals, focusing initially on restoring autonomy for those with severe neurological conditions. The device itself, about the size of a coin, is implanted into the skull and features ultra-fine electrode “threads” that are delicately inserted into the brain’s cortex by a precision surgical robot.
These threads are positioned near neurons to detect action potentials, the tiny electrical signals that represent intended movements, feelings, or thoughts. For patients with paralysis, this technology offers a revolutionary pathway to bypass a damaged spinal cord or nervous system. It enables them to control a computer cursor, type, and interact with the digital world using purely cerebral commands.
The successful first human implant in January 2024 marked a pivotal moment, transitioning the Neuralink brain chip from a high-profile research project into an active clinical device. This development highlights why the idea of a brain-computer connection is no longer fantastical but a rapidly advancing medical and technological frontier, ushering in the age of genuine thought controlled devices. The foundational work being done now, including the initial brain chip clinical trials, is not just about helping patients but is laying the groundwork for future cyborg human augmentation.
Brain-Computer Interface (BCI) Simply Explained
At its core, a brain-computer interface technology (BCI), or brain-machine interface (BMI), is a direct communication pathway between the brain’s electrical activity and an external device. Think of it as a sophisticated translator. The brain communicates through electrical impulses generated by neurons. When you decide to move your hand, a specific pattern of electrical activity occurs in the motor cortex. A BCI like the Neuralink brain chip intercepts and “reads” these specific patterns.
The system then uses sophisticated algorithms to translate those electrical signatures—your “intent”—into a clear, digital command, such as “move cursor up” or “click.” Unlike non-invasive systems (like EEG caps worn on the scalp), invasive BCIs, such as the one developed by Neuralink, are surgically placed inside or on the brain tissue. This proximity to the neurons allows for the acquisition of much clearer, higher-resolution neural data, which is crucial for achieving fine, precise control over external devices. This direct, high-fidelity link is what makes the Neuralink brain chip so potent and why the term “cyborg” now applies to individuals using these thought controlled devices to interact with the world.
Why Neuroimplants and “First Cyborgs” Were Needed in the First Place
The primary, and most profound, driver behind the development of neuroimplants like the Neuralink brain chip is the immense unmet need of individuals living with severe neurological impairments. For people with quadriplegia (loss of movement in all four limbs) due to spinal cord injury, or neurodegenerative diseases like Amyotrophic Lateral Sclerosis (ALS), the connection between the brain and the body’s muscles is broken. Their cognitive function remains intact—they can think about moving—but the signal never reaches the limbs. BCIs are designed to bridge this gap. By enabling a person with paralysis to control a computer or a robotic arm with only their thoughts, the technology restores critical levels of communication, mobility, and independence.
The label of “first cyborgs” highlights this transformative restoration of function; it’s not about adding a new feature, but about retrieving lost autonomy. Furthermore, the data gathered from these initial brain chip clinical trials using the Neuralink brain chip is invaluable, accelerating research into treatments for a wider range of neurological conditions, including memory loss, depression, and blindness, pushing the boundaries of medical possibility and solidifying the necessity of this advanced brain-computer interface technology.
Curious how advanced human–machine synergy looks on four wheels instead of inside the body? Check out the Zeekr 7 GT, a brutal 800V electric GT tuned for European roads and WLTP range tests: https://autochina.blog/zeekr-7-gt-review-europe-wltp-800v-charging/ and compare tomorrow’s cyborg drivers with their EVs in real-world performance, pricing, comfort, and technology metrics.

2. How the Brain-Computer Interface (BCI) Works Using the Neuralink brain chip as an Example
The functioning of a Neuralink brain chip system is a sophisticated interplay of cutting-edge hardware, delicate surgical robotics, and advanced artificial intelligence (AI) algorithms. The entire system is comprised of three primary components: the implant itself (the N1 Implant), the surgical robot (the R1 Robot), and the external application/decoder software. The N1 Implant, which is placed flush with the skull, houses the ultra-fine electrode threads—each finer than a human hair—that extend into the brain’s motor cortex.
When a user with the Neuralink brain chip intends to perform an action, such as moving a cursor, the neurons in the motor cortex fire, generating a distinct electrical signature. These tiny electrical signals are picked up by the 1,024 electrodes on the threads.
The implant’s custom, low-power chips and electronics then process and amplify these neural signals before transmitting the data wirelessly, using a Bluetooth-based protocol, to a connected device like a smartphone or tablet. The device runs the Neuralink Application, which contains the critical decoding algorithms. This process is the core of brain-computer interface technology, effectively translating a patient’s neural intent into an actionable digital command. This robust and high-resolution data acquisition is what sets the Neuralink brain chip apart, allowing for control that is precise and responsive, making it one of the most promising thought controlled devices available today.
Electrical Signals of the Brain and Their “Translation” into Commands
The brain operates on an electrochemical language. Neurons communicate by generating short electrical pulses called action potentials or “spikes.” The Neuralink brain chip works by recording the collective activity of many individual neurons near its implanted threads. When a person thinks about moving their hand, a predictable and repeatable pattern of these action potentials occurs in the motor cortex. The goal of the BCI system is to capture this pattern and map it to a specific external action. This mapping is the “translation.” The raw electrical data—the recorded neural spikes—are transmitted wirelessly to the external processing unit.
Here, sophisticated AI algorithms, specifically machine learning models, are trained to recognize the distinct neural patterns associated with different intentions. For example, a certain pattern might be associated with “move cursor right,” while another might mean “click.” Through extensive training and calibration, the system learns to accurately and rapidly decode the user’s intent. This rapid and accurate decoding is what transforms the neural signal into a functional digital command, providing the foundation for precise thought controlled devices. The success of the Neuralink brain chip hinges on the quality of the neural data it captures and the intelligence of the decoding algorithms that interpret it.
Decoding Thoughts: Cursor, Clicks, and Basic Actions
The initial, and profoundly impactful, application of the Neuralink brain chip involves decoding basic, motor-related thoughts for digital control. The earliest patient, Noland Arbaugh, demonstrated his ability to control a computer cursor, click, and play video games solely with his thoughts. This is achieved by the BCI algorithms recognizing and mapping the neural patterns corresponding to intended motor movements, such as the thought of moving a hand or wrist.
This technology is a form of neuroprosthetics, bypassing the paralyzed limbs to restore a crucial communication pathway. For a person with high-level quadriplegia, gaining the ability to move a cursor and click—essential functions for using a smartphone or computer—is life-changing. It restores the capacity to communicate, access information, use banking services, and engage in social media.
The system for the Neuralink brain chip is continuously learning and refining its decoding, ensuring the user’s control becomes more fluid and intuitive over time. While the current applications are focused on restoring fundamental digital control, the high-resolution nature of the device suggests future potential for more complex commands, which is why the brain chip clinical trials are such a critical component of testing this evolving brain-computer interface technology.
Limitations of Current BCIs and Where They Are Still Weak
Despite the groundbreaking progress of the Neuralink brain chip and other invasive BCIs, the technology still faces significant limitations that must be addressed for widespread adoption. One key challenge is the long-term biological stability and safety of the implant itself. The brain’s immune response can lead to the formation of glial scar tissue around the electrodes over time, which can degrade the quality of the neural signal recording—a phenomenon known as signal drift or attenuation. While Neuralink’s flexible threads are designed to minimize this, the long-term viability of high-resolution recordings remains a critical area of research.
Another limitation is the complexity of the signal decoding. Current BCIs are highly effective at translating motor intent—the thought of moving a body part—but are far from being able to “read” complex, abstract thoughts, or emotions with high fidelity. Furthermore, the necessity of invasive brain surgery, even with a precision robot, carries inherent risks, which limits the technology primarily to individuals with severe medical conditions (i.e., BCI implants for paralysis). Finally, the ethical and regulatory frameworks are still catching up with the speed of innovation, creating uncertainty regarding neurotechnology ethics and privacy, especially concerning who owns the sensitive neural data collected by the Neuralink brain chip.
3. Neuralink brain chip: History of the Project and Key Development Stages
The journey of the Neuralink brain chip from a conceptual ambition to a functional implantable device has been one of rapid, highly visible development, largely fueled by the vision and resources of its founder, Elon Musk. Established in 2016, the company initially operated in relative secrecy, recruiting top talent from the fields of neuroscience, chemistry, and engineering. The core objective was to create an ultra-high bandwidth, fully implantable brain-computer interface technology that could not only restore lost function but eventually facilitate cyborg human augmentation. The first major public demonstration occurred in 2019, showcasing the ultra-fine electrode threads and the highly complex surgical robot necessary for their precise, atraumatic implantation.
This stage focused on demonstrating the core components of the system. The subsequent years involved extensive preclinical testing, primarily on animals, to prove the device’s safety, biocompatibility, and functionality in reliably recording neural signals. This rigorous testing was crucial for paving the way toward human brain chip clinical trials. The relentless pursuit of a seamlessly integrated, cosmetically invisible device—powered wirelessly and capable of high-data-rate transmission—set a new benchmark for thought controlled devices. The history of the Neuralink brain chip is characterized by an engineering-first approach, prioritizing the miniaturization and automation necessary for a scalable medical device.
From First Prototypes to Thousands of Hours of Tests
The early prototypes of the Neuralink brain chip were focused on refining the key technological innovations: the flexible electrode threads and the surgical robot. Traditional BCI arrays are more rigid and can cause micromotion relative to the brain tissue, potentially leading to scarring and signal degradation. Neuralink’s threads, however, are ultra-flexible and are designed to minimize damage during insertion and subsequent movement.
Developing the R1 Surgical Robot was equally pivotal; a human hand cannot reliably insert these delicate threads. The robot uses advanced optics and imaging (including Optical Coherence Tomography or OCT) to precisely guide the needle, inserting up to six threads (with 192 electrodes) per minute, avoiding surface vasculature. Before moving to human trials, the Neuralink brain chip underwent thousands of hours of rigorous testing in preclinical studies, primarily on pigs and non-human primates. These studies confirmed the device’s ability to safely record high-fidelity neural data over extended periods and decode movement intent.
The data from these tests were essential for demonstrating the system’s stability and accuracy to regulatory bodies, culminating in the 2023 FDA clearance for human brain chip clinical trials, solidifying the foundation of this revolutionary brain-computer interface technology.
Implantation in at least 12 People and 15,000+ Hours of Use
As of late 2025, the Neuralink brain chip has reportedly been implanted in at least twelve human participants as part of the PRIME (Precise Robotically Implanted Brain-Computer Interface) Study. This early feasibility study, which seeks patients with quadriplegia (including those with spinal cord injury or ALS), is designed to assess the safety of the N1 Implant and the R1 Surgical Robot, as well as the initial functionality of the BCI system for enabling thought controlled devices.
The most publicly known pioneer, Noland Arbaugh, a quadriplegia patient, received his implant in January 2024. He has since logged thousands of hours using the device to play games, use social media, and browse the web, showcasing unprecedented levels of control.
Other pioneers, such as Alex and Brad, have also been actively using their implants for communication and creative tasks. The cumulative use of the Neuralink brain chip across all participants is rapidly approaching or has exceeded 15,000 hours, providing a massive, invaluable dataset on long-term device performance, signal stability, and the real-world utility of the BCI implants for paralysis. The consistent, real-time data being gathered from these ongoing brain chip clinical trials is crucial for iterating the decoding algorithms and ensuring the long-term safety and efficacy of the Neuralink brain chip.
The Role of Elon Musk and Why There is So Much Hype Around Neuralink
Elon Musk’s involvement is arguably the single greatest factor driving the intense public interest and “hype” surrounding the Neuralink brain chip. Musk’s reputation for taking on “impossible” engineering challenges (SpaceX, Tesla) and his public-facing style generate immense media attention. His candid discussions about the long-term vision—beyond just medical restoration—of achieving cyborg human augmentation and a symbiotic relationship with AI have captured the public imagination. Musk often frames the Neuralink brain chip as a necessity for humanity to keep pace with advancing artificial intelligence. This grand, futuristic narrative elevates Neuralink beyond a typical medical device company.
While this high-profile status accelerates funding and talent acquisition, it also subjects the company to intense scrutiny. Critics often point to the gap between Musk’s ambitious rhetoric and the current, more modest, clinical reality (which is primarily focused on restoring cursor control for BCI implants for paralysis). Regardless of the debate, Musk’s role ensures the Neuralink brain chip remains at the forefront of the global conversation on neurotechnology, accelerating the entire field of brain-computer interface technology and drawing unprecedented attention to the importance of thought controlled devices.

4. The First “True Cyborgs”: Real-World Cases of Neuralink brain chip Users
The term “cyborg” can be complex, but in the context of the Neuralink brain chip, it refers to the literal integration of a machine—the BCI device—with the human nervous system to restore or augment function. The individuals participating in the PRIME study are the world’s first Neuralink pioneers, and they represent the real-world application of this groundbreaking brain-computer interface technology. These patients, all living with conditions like quadriplegia or ALS, have had their ability to interact with the world fundamentally transformed.
Their personal stories and the functionality demonstrated in their daily lives prove that the Neuralink brain chip is not a laboratory curiosity but a functioning neuroprosthetic. The cases highlight the dramatic shift in autonomy that comes with controlling digital devices directly with neural signals.
The public demonstrations, which often show patients engaging in activities that were previously impossible, like playing complex video games or coding, provide compelling evidence of the system’s effectiveness. These pioneers are the first wave of people who are directly integrated with technology, effectively becoming the cyborg human augmentation test cases that will define the future of the field.
Cursor Control Using the Power of Thought
The most significant and immediate outcome demonstrated by the Neuralink brain chip pioneers is the ability to achieve precise, high-speed control of a computer cursor using only thought. Noland Arbaugh, the first recipient, was shown moving the cursor across a screen, clicking, dragging, and dropping items simply by intending the movement, bypassing his paralyzed limbs entirely. The high-resolution data provided by the 1,024 electrodes enables a level of cursor control fidelity that is crucial for practical, daily use.
Unlike previous systems, which might have struggled with speed or accuracy, the Neuralink system allows users to perform tasks efficiently, such as typing text, using a web browser, or navigating complex digital interfaces. This restored digital access is a profound game-changer for individuals with severe mobility impairments, as the ability to operate a computer or smartphone is a primary key to communication, education, and employment in the modern world. This success in high-fidelity control with the Neuralink brain chip proves the viability of its core brain-computer interface technology for the most common digital tasks.
Robotic Arms and Prostheses That Obey the Brain
Beyond digital screens, the Neuralink brain chip is actively being tested for controlling physical, external prosthetics and robotic arms. This application is the ultimate goal of BCI implants for paralysis: restoring physical interaction with the environment. In late 2025, Alex Conley, another PRIME study participant, was reported to be the first to use the Neuralink device to control a robotic arm. The principle is the same as cursor control: the patient intends a movement (e.g., “reach for the cup”), the brain generates the corresponding neural pattern, and the Neuralink brain chip records, decodes, and transmits this intent, but this time, the output command is directed to the motors of a robotic arm.
The challenge here is far greater than cursor control, requiring a much more nuanced and rapid decoding of movement in three-dimensional space, including grasp, rotation, and force control. Successfully linking the brain’s motor intent directly to complex robotic movements represents a monumental leap in the field of neuroprosthetics and is a tangible example of advanced thought controlled devices being integrated into daily life.
Control of Gadgets and Devices “Hands-Free” — Only with the Brain
The future application of the Neuralink brain chip extends to controlling a wide ecosystem of smart devices and gadgets in the user’s environment, moving towards true cyborg human augmentation of daily function. By integrating with protocols like Bluetooth and utilizing the BCI-Human Interface Device (BCI-HID) protocols being developed, the implant can theoretically send direct commands to any compatible device. This means a user could potentially turn on lights, adjust a thermostat, stream music, or answer a phone call simply by thinking the command, eliminating the need for voice commands, physical switches, or even eye-tracking. The Neuralink brain chip essentially serves as a universal remote control powered by the mind.
This “hands-free” control of the environment not only enhances the independence of individuals with motor impairments but also opens the door to future applications in the general population, such as enhanced gaming or augmented reality interfaces. The ongoing brain chip clinical trials are crucial for refining the system’s ability to handle this diverse range of commands accurately and reliably in a real-world setting.
5. Neuralink’s Competitors: Synchron, Blackrock Neurotech, and Other BCI Players
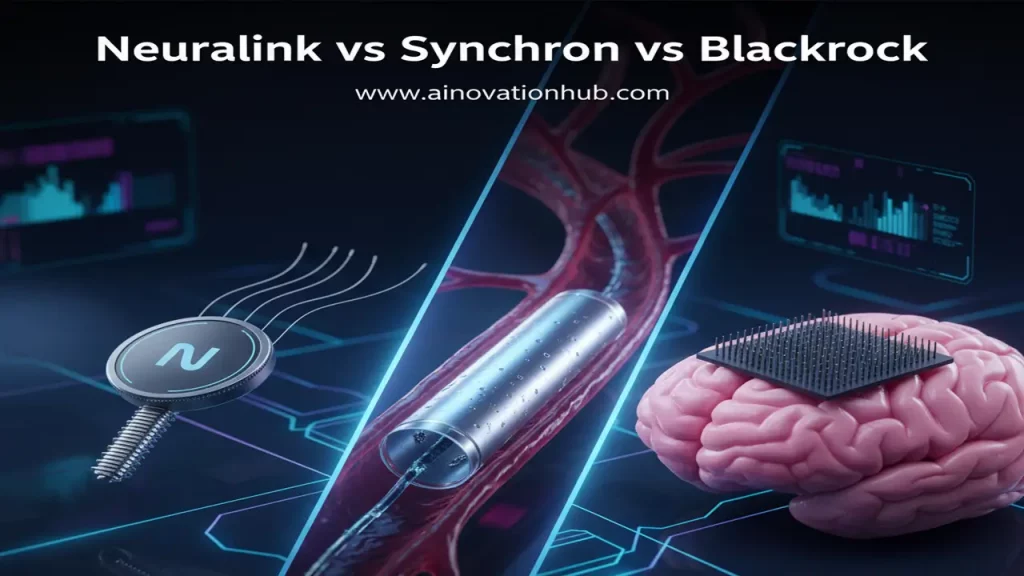
While the Neuralink brain chip has captured the most public attention, it is crucial to recognize that the field of brain-computer interface technology is a vibrant, competitive ecosystem with several established and innovative players. Companies like Synchron and Blackrock Neurotech have significant clinical experience and different technological approaches that present viable alternatives, particularly for BCI implants for paralysis patients. The competition is not just about technology but also about surgical approach, safety profile, and regulatory approval, which are all key to market adoption. This competitive landscape is driving rapid innovation, as each company pushes the boundaries of neural recording fidelity, surgical invasiveness, and long-term device stability.
The primary competition revolves around the trade-off between the invasiveness of the procedure and the fidelity (resolution) of the neural data captured. For instance, less invasive methods, while safer, typically yield lower-resolution signals, while more invasive ones, like Neuralink’s, provide high-fidelity data but require open-brain surgery. The diverse approaches being pursued by these major players are collectively advancing the state-of-the-art in thought controlled devices.
Synchron and Its Stent-Implant in Brain Vessels
Synchron brain computer interface is a key competitor known for its minimally invasive approach. Their flagship device, the Stentrode™, is a fully implantable BCI that utilizes an endovascular method. Instead of requiring open-brain surgery, the Stentrode is delivered through the jugular vein in a procedure similar to a standard cardiac stent placement. Once navigated to the motor cortex, the device’s electrodes rest inside the blood vessel, next to the brain tissue, to record neural signals.
This dramatically reduced surgical risk is a significant advantage for patients and makes it a more scalable option for BCI implants for paralysis. The Stentrode system has been tested in clinical trials and has demonstrated the ability to allow users to control digital devices like smartphones and computers.
While its placement inside a blood vessel means the quality of its neural signal recording may be slightly lower than an intracortical implant like the Neuralink brain chip, the reduced invasiveness presents a compelling safety and accessibility profile. Synchron was also the first BCI company to co-develop and integrate with Apple’s BCI-Human Interface Device (BCI-HID) protocol, allowing direct brain control of iOS devices, showcasing their commitment to real-world utility of their brain-computer interface technology.
Blackrock Neurotech and Their Deep Neural Implants
Blackrock Neurotech brain implant technology is one of the most clinically proven BCI platforms in the world. Their core technology, the Utah Array, has been used in human clinical studies for nearly two decades. Unlike Neuralink’s flexible threads, the Utah Array is a microelectrode array (a small grid of silicon-based microelectrodes) that is surgically implanted directly into the brain’s cortex.
This invasive approach offers extremely high-resolution, high-fidelity neural recordings, which has enabled users to achieve remarkable feats, such as typing at up to 90 characters per minute and decoding up to 62 words per minute from thought, or controlling advanced robotic arms for painting and using complex software like Photoshop. Blackrock Neurotech holds the distinction of having the most human patients implanted with their BCI technology globally, offering over 19 years of human study experience.
Their MoveAgain BCI System, which received FDA Breakthrough Device designation, is designed to provide greater independence to patients with motor impairment. The long-term track record and proven high-fidelity recording capability make Blackrock Neurotech brain implant a gold standard in invasive brain-computer interface technology, offering a mature, alternative pathway to thought controlled devices alongside the highly publicized Neuralink brain chip.
Other Companies and the Global Map of Clinical Trials
The BCI landscape extends far beyond the major players of the Neuralink brain chip, Synchron, and Blackrock Neurotech. Numerous academic institutions and smaller start-ups are conducting a global array of brain chip clinical trials, each focusing on specialized aspects of brain-computer interface technology. For instance, some research is focused on ECoG (Electrocorticography) arrays, which are placed on the surface of the brain (partially invasive), offering a compromise between the high-resolution of deep implants and the safety of non-invasive systems. Other trials are specifically targeting the restoration of speech by decoding neural signals related to the intended formation of words, or restoring vision through direct stimulation of the visual cortex.
The global map of BCI clinical trials is vast, encompassing a variety of targets (motor cortex, somatosensory cortex, speech centers) and applications (restoring movement, communication, sensation). This expansive network of research ensures that advancements are rapid and diverse, with continuous pressure on all players, including the developers of the Neuralink brain chip, to innovate and improve the long-term safety, utility, and accessibility of BCI implants for paralysis and other neurological conditions.

6. Hardware and Software: What the Modern Neuralink brain chip and BCI System Consist Of
The modern Neuralink brain chip and its accompanying BCI system represent a marvel of miniaturized, integrated engineering. Far from being a simple recording device, it is a complex, wirelessly powered system designed to operate reliably within the harsh, challenging environment of the human body for decades. The system is a complete loop: it acquires tiny electrical signals, processes them in real-time, translates them into digital commands using intelligent algorithms, and transmits the results wirelessly. This sophistication in hardware, especially the hermetically sealed implant and the ultra-fine electrodes, is what enables the high-bandwidth, high-fidelity communication necessary for precise control.
Furthermore, the reliance on specialized AI software for signal decoding is equally critical. The seamless integration of these physical and digital components is what distinguishes the Neuralink brain chip as a next-generation brain-computer interface technology, moving beyond the bulkier, wired systems of the past toward a fully integrated, cosmetically invisible solution for thought controlled devices.
The Implant, Electrodes, and the Surgical Robot
The hardware core of the system is the N1 Implant itself. It is a small, disc-shaped device that is hermetically sealed in a biocompatible enclosure to protect the internal electronics from the body’s fluids. The implant contains a battery, custom, low-power Application-Specific Integrated Circuits (ASICs) for processing and amplification of neural signals, and the wireless communication components. The implant connects to 1,024 electrodes housed on highly flexible, ultra-thin “threads” (each thinner than a human hair) made of polyimide. These threads are the critical interface, inserted directly into the motor cortex to detect action potentials from surrounding neurons. The implantation process for the Neuralink brain chip is performed entirely by the R1 Surgical Robot.
The robot uses advanced machine vision and micron-level precision to insert the threads, ensuring they are placed accurately and without damaging the brain’s surface blood vessels. This automated surgical approach is a significant innovation, designed to ensure the procedure is safe, rapid, and reproducible—a necessary condition for scaling up brain chip clinical trials and eventual commercial use of the Neuralink brain chip.
AI Algorithms That Learn to “Read” Thoughts
The software, particularly the NeuroAI algorithms, is where the “magic” of the Neuralink brain chip truly happens. The raw neural data collected by the electrodes—a torrent of electrical spikes—is meaningless without a decoder. The BCI system employs advanced machine learning algorithms to interpret this complex data stream. During a calibration phase, the user thinks about performing specific actions (e.g., moving a cursor up/down), and the AI algorithms learn to correlate the resulting unique neural patterns with those specific movement intents. This process is known as decoding. Because the neural signals can change over time (due to subtle movement or biological changes), the algorithms in the Neuralink brain chip are designed to be adaptive.
They continuously learn and refine their models to maintain high accuracy and stability, ensuring the user’s control over the thought controlled devices remains fluid and reliable. The success of the Neuralink brain chip in real-time control, such as playing fast-paced video games, is a testament to the speed and sophistication of these AI-driven decoding algorithms.
Wireless Data Transmission and Connection Security
A key feature of the Neuralink brain chip is its fully wireless operation, which is critical for achieving a cosmetically invisible and user-friendly BCI system. The implant is powered by a small battery that is recharged wirelessly from the outside via a compact, inductive charger, similar to how a modern smartphone or smartwatch is charged. The processed neural data is also transmitted wirelessly to the external device (e.g., a tablet or phone) via a high-speed, secure link (likely a customized Bluetooth protocol).
Ensuring the security and integrity of this connection is paramount, particularly given the highly sensitive nature of the data being transmitted. The system must employ robust encryption and data protection protocols to prevent unauthorized access or interception of the neural signals. This focus on secure, high-bandwidth wireless transmission makes the Neuralink brain chip a practical, real-world brain-computer interface technology capable of supporting continuous, high-performance use of thought controlled devices in the user’s everyday environment.

7. Benefit vs. Risks: Health, Side Effects, and the Ethics of Cyborgization
The advent of highly advanced, invasive BCIs like the Neuralink brain chip brings into sharp focus a critical debate: the immense potential for human benefit versus the inherent medical and ethical risks of integrating technology directly into the brain. For patients with severe paralysis, the benefit of restored autonomy is immeasurable. However, the technology is not without risk. The surgical procedure itself, the potential for device failure, long-term biological reactions, and the profound questions of neurotechnology ethics and privacy must be carefully considered.
Regulators, developers (including those of the Neuralink brain chip, Synchron brain computer interface, and Blackrock Neurotech brain implant), and ethicists must collaborate to establish frameworks that maximize patient benefit while rigorously protecting human subjects and their most intimate data. The conversation must move beyond the “hype” to a grounded assessment of the long-term trade-offs inherent in this form of cyborg human augmentation.
Who the Neuralink brain chip Truly Helps Now (Paralysis and Severe Diseases)
Currently, the Neuralink brain chip and similar devices from competitors are primarily focused on helping individuals with severe neurological impairments who have exhausted other options. The main target population for BCI implants for paralysis includes those with quadriplegia due to spinal cord injury or advanced neurodegenerative diseases like ALS. For these individuals, the Neuralink brain chip offers a way to bypass the damaged nerve pathways and regain control over digital devices, thus restoring communication, productivity, and social engagement. Patients like Noland Arbaugh are testaments to the life-altering potential of the technology—the ability to independently control a computer or a robotic arm is a fundamental restoration of autonomy, not a luxury.
While the long-term vision of the Neuralink brain chip involves cyborg human augmentation for the general public, its immediate, life-changing utility is firmly rooted in the medical treatment of the most vulnerable patient populations through advanced thought controlled devices.
Operational Risks, Device Failures, and Long-Term Consequences
The primary risks associated with the Neuralink brain chip and all invasive BCI implants begin with the surgical procedure. Although Neuralink uses a highly precise robot (the R1) to minimize risks, any open-brain surgery carries the inherent dangers of infection, bleeding, or brain tissue damage.
Furthermore, the long-term reliability and biological response of the implanted device are significant concerns. Issues such as signal degradation due to gliosis (scar tissue formation around the electrodes), device migration, or electrical failure of the hardware over decades of use must be thoroughly investigated during brain chip clinical trials. The case of Neuralink’s first patient experiencing thread retraction, which led to a decrease in performance before being addressed by algorithmic improvements, underscores the technical challenges of long-term stability.
Researchers must continually monitor for potential long-term consequences, including the possibility of localized inflammation or unanticipated side effects from chronic electrical recording and stimulation.
Issues of Thought Privacy and Control Over Brain Data
Perhaps the most profound risk is ethical, centering on neurotechnology ethics and privacy. BCI devices, especially those with high-resolution recording capabilities like the Neuralink brain chip, capture neurodata—information about a person’s thoughts, intentions, and even emotional states. This is arguably the most intimate data imaginable. The ethical concerns are manifold:
- Mental Privacy: Who owns this neural data? How will it be protected from hackers, commercial exploitation, or government surveillance? The UNESCO has adopted the first global standard on neurotechnology ethics to address these very risks, emphasizing the inviolability of the human mind.
- Consent and Autonomy: Can a patient truly give informed consent for a technology whose long-term effects are unknown? Furthermore, as BCIs potentially advance to stimulate or influence the brain, there is a risk of external interference challenging an individual’s freedom of thought and cognitive liberty.
- Security: The wireless transmission of data from the Neuralink brain chip presents a new attack vector. Any security vulnerability could expose the user’s thoughts or, in the future, allow unauthorized parties to send harmful or manipulative commands back into the device.
These issues necessitate stringent regulation and transparent development to ensure this new wave of thought controlled devices respects human rights and dignity.

8. From Lab to Mass Market: Clinical Trials and Regulatory Approval
The path for the Neuralink brain chip and its competitors from a promising laboratory prototype to a widely available commercial device is governed by a rigorous, multi-stage process of clinical trials and regulatory oversight. This trajectory is essential to ensure that any new brain-computer interface technology is safe, effective, and ethically sound for human use.
The current stage, involving early feasibility studies like Neuralink’s PRIME trial, is focused on fundamental safety and initial functionality, primarily for BCI implants for paralysis. Moving to a mass-market product requires progressing through larger, pivotal trials that demonstrate long-term efficacy and address a broader range of clinical needs. The ultimate gatekeeper for market access is the regulatory body, such as the U.S. Food and Drug Administration (FDA), whose approval process is designed to balance the potential for life-changing medical breakthroughs with the imperative to protect public health. The speed at which devices like the Neuralink brain chip advance depends entirely on the outcomes of these brain chip clinical trials and the willingness of regulators to create appropriate, forward-looking guidelines.
Why Dozens of BCI Clinical Trials Are Needed Globally
Dozens of global brain chip clinical trials for BCIs are necessary because no single device or approach provides a universal solution. Each clinical trial serves a unique purpose:
- Safety and Biocompatibility: Early-stage trials (like Neuralink’s PRIME) focus on the acute safety of the implant procedure and the device itself (e.g., the Neuralink brain chip or the Synchron brain computer interface). They assess risks such as infection, hemorrhage, and initial tissue reaction.
- Efficacy for Specific Conditions: Later trials must demonstrate that the BCI reliably achieves its intended function—for example, that the device can sustain high-fidelity cursor control or decode speech intent over many years. Different neurological conditions (ALS, spinal cord injury, stroke) require tailored BCI solutions.
- Long-Term Stability: Crucially, trials must prove the long-term stability of the neural signal recording and the device’s functional integrity over a lifetime, which requires years of follow-up on implanted subjects.
- System Refinement: The trials provide real-world data that developers use to continuously refine the AI decoding algorithms and improve the overall user experience for thought controlled devices. This diversity and depth of research are vital for the responsible development of brain-computer interface technology for BCI implants for paralysis and beyond.
The Role of the FDA and Other Regulators
The U.S. FDA plays a paramount role in the commercialization of the Neuralink brain chip and all medical BCIs. As an investigational medical device, the BCI must first receive an Investigational Device Exemption (IDE) approval from the FDA to begin human trials, which Neuralink received in 2023. This is followed by a rigorous, multi-step review process:
- Early Feasibility Studies (EFS): Small studies to gather initial data on safety and function (the current stage of the Neuralink brain chip).
- Pivotal Trials: Larger, well-controlled studies designed to definitively prove the device is effective and safe for its intended use.
- Pre-Market Approval (PMA): The final, comprehensive application required for Class III (high-risk) medical devices like invasive BCI implants before they can be legally marketed in the U.S.
International regulators, such as the European Medicines Agency (EMA), have similar stringent requirements. The FDA’s role is not just to approve the technology but also to weigh the clinical benefit against the risks and to work with developers and ethicists to address the unique challenges of neurotechnology ethics and privacy. Regulatory caution is a necessary safeguard to prevent premature release of a complex and highly invasive technology like the Neuralink brain chip.
Realistic Timelines: When These Implants Might Be Available to Ordinary People
Based on the typical trajectory of Class III medical devices, it is highly likely that BCI implants for paralysis will become commercially available for patients with the most severe medical needs within the next 3-7 years (roughly 2028-2032), assuming current brain chip clinical trials remain successful and meet all regulatory milestones. This initial availability will be restricted to therapeutic uses. The timeline for the Neuralink brain chip and similar devices to be widely available for cyborg human augmentation for ordinary people (i.e., for non-medical purposes like cognitive enhancement or enhanced gaming) is much further out, likely 10-20 years or more. This future timeline is dependent on several factors:
- Long-Term Safety: Decades of follow-up data will be required to convince regulators and the public that the surgical risks are justified for non-medical use.
- Cost and Scalability: The complex surgical procedure and high-tech hardware must become significantly more affordable and scalable.
- Ethical Consensus: A broad societal and regulatory consensus on neurotechnology ethics and privacy must be established to permit the elective, non-therapeutic implantation of thought controlled devices. The medical restoration of function must precede the augmentation of function.

9. H2: Cyborgs in Daily Life: How the Neuralink brain chip Will Change Work, Gaming, and Communication
The successful translation of the Neuralink brain chip from a therapeutic device for BCI implants for paralysis into a platform for general cyborg human augmentation will profoundly disrupt numerous aspects of daily life. By establishing a direct, high-bandwidth communication channel between the human cortex and digital technology, the Neuralink brain chip promises to remove the latency and limitations inherent in traditional user interfaces (keyboards, mice, touchscreens).
This shift will redefine how humans interact with technology, leading to new professional capabilities, unprecedented levels of immersion in gaming, and entirely new modalities of communication. While the initial focus remains on medical restoration, the underlying brain-computer interface technology will eventually unlock a future where human intention is instantly translated into digital action, leading to a new era of “mind-mediated” digital interaction.
Future Professions: Cyborg Operators, Cyber-Sports, Design, Finance
The introduction of thought controlled devices will create entirely new professional roles and fundamentally alter existing ones.
- Cyborg Operators: In fields requiring extreme precision and speed, such as remote surgery, complex drone operation, or advanced manufacturing, direct neural control via the Neuralink brain chip could make “cyborg operators” significantly faster and more precise than their unaugmented counterparts.
- Cyber-Sports and Gaming: The latency advantage of direct neural input would likely create a new tier of elite “cyber-athletes” in video games, who can execute complex maneuvers at the speed of thought.
- Design and Finance: Designers could “think” a 3D model into existence or manipulate complex CAD software with unprecedented fluidity. In high-frequency trading or complex financial modeling, the ability to instantly process and execute commands via a brain-computer interface technology could give a distinct professional advantage. This potential for a “cognitive performance boost” raises significant questions about workplace ethics and equality, which are critical elements of the neurotechnology ethics and privacy debate.
Gaming and AR/VR with a Direct Connection to the Brain
The most immediately compelling non-medical application of the Neuralink brain chip may be in immersive entertainment. Direct neural connection would revolutionize the gaming and Augmented Reality (AR)/Virtual Reality (VR) experience:
- Zero-Latency Control: Imagine controlling an in-game avatar or vehicle with the speed of thought, eliminating the need for controllers and achieving truly intuitive interaction.
- Augmented Immersion: The BCI could work bidirectionally, potentially allowing the transmission of simulated sensory feedback directly to the brain, leading to a level of AR/VR immersion that is currently impossible. Thinking of a virtual object could bring it into focus, or a neural command could switch between digital environments. This direct brain-to-digital interface transforms the very nature of human-computer interaction. The high-bandwidth nature of the Neuralink brain chip is specifically designed for this level of rapid data flow, positioning it as a key enabling technology for the next generation of truly immersive and thought controlled devices in entertainment.
Social Effects: Inequality of Access, “Augmented” People, and New Rules of the Game
The mass adoption of BCI for cyborg human augmentation will inevitably trigger profound social and ethical consequences.
- Access Inequality: If the Neuralink brain chip or similar devices provide a significant cognitive or professional advantage, limited access (due to high cost or complex procedures) could create a stark divide between “augmented” and “unaugmented” individuals, exacerbating existing social and economic inequalities.
- Redefining “Normal”: The concept of human ability, cognitive function, and even intelligence could be redefined. Society would have to grapple with the implications of an augmented class of people.
- New Rules: New ethical and legal rules will be required to govern human-computer interaction, especially regarding the use of neural data in public or professional settings. These are central tenets of the neurotechnology ethics and privacy debate. For instance, should an augmented individual have an unfair advantage in a competitive environment? Should a company be allowed to require a Neuralink brain chip for employment? Addressing these complex social questions is as important as the technological development itself.

10. Conclusion: Will We Become Cyborgs, and What Role Will the Neuralink brain chip Play in the Future of AI?
The question is no longer if we will become cyborgs, but when and how. The work of companies like Neuralink, Synchron, and Blackrock Neurotech has made the integration of advanced brain-computer interface technology into the human body a medical reality today and a potential societal reality tomorrow.
The Neuralink brain chip is currently the most high-profile engine driving this change, providing critical proof-of-concept for high-resolution, wireless, implantable thought controlled devices. Its initial success in enabling BCI implants for paralysis patients to control computers with their minds marks a crucial milestone—the therapeutic application of the technology is established. However, the path to mass cyborg human augmentation is still long and complex, governed not only by engineering breakthroughs but by ethical caution and regulatory oversight. The BCI revolution is inherently intertwined with the future of Artificial Intelligence; the Neuralink brain chip is essentially a new, high-bandwidth input/output port for the human brain to interact with AI models, making it a critical piece of the future human-AI ecosystem.
What Has Already Been Proven, and What Remains Hype
Proven Reality:
- The Neuralink brain chip can safely be implanted using a precision robot (the R1).
- High-resolution neural signals can be recorded from the human motor cortex wirelessly and continuously.
- Individuals with severe paralysis can reliably control cursors, click, type, and even control robotic arms with their thoughts, demonstrating functional restoration using thought controlled devices.
- The core brain-computer interface technology is clinically viable.
What Remains Hype/Future Goals:
- Mass-market cyborg human augmentation for non-medical purposes (e.g., enhanced memory, telepathy) remains purely aspirational and is many years away.
- The ability to “read” complex, abstract thoughts or implant memory with high fidelity has not been proven.
- The long-term safety (10+ years) and the absence of signal degradation in the Neuralink brain chip and its threads must still be definitively proven in ongoing brain chip clinical trials.
How to Follow the Development of BCI and Neuroimplants Without Panic
To follow the rapid development of the Neuralink brain chip and other neuroimplants responsibly, it is essential to focus on clinical trial data and official regulatory announcements rather than sensational headlines or founder rhetoric.
- Check Official Sources: Follow updates from the FDA, and look for peer-reviewed publications from the clinical teams involved with the Neuralink brain chip, Synchron brain computer interface, and Blackrock Neurotech brain implant.
- Focus on Function, Not Fantasy: Pay attention to what patients are actually able to do (e.g., characters per minute typed, cursor accuracy) versus the long-term, non-medical speculation.
- Engage with Ethics: Keep abreast of discussions regarding neurotechnology ethics and privacy from bodies like UNESCO and the Global Privacy Assembly. The ethical framework will dictate the pace and scope of future development more than the technology alone.
Soft CTA with an invitation to read fresh neurotechnology analyses on www.aiinovationhub.com.
The journey into the age of the cyborg is just beginning, and the Neuralink brain chip is a central figure in this unfolding story. Understanding its progress, its ethical challenges, and its role in the competitive landscape of brain-computer interface technology is key to grasping the future of human potential.
Would you like to stay ahead of the curve and read the latest, in-depth analyses of neurotechnology and AI? Visit www.aiinovationhub.com for our fresh, expert-driven content.
Related
Discover more from AI Innovation Hub
Subscribe to get the latest posts sent to your email.